N- Hexane
Product Details:
- Chemical Name N- Hexane
- CAS No 110-54-3
- Physical State Liquid Coating
- Usage For Lab
- Purity(%) 99%
- Click to view more
X
N- Hexane Price And Quantity
- 200 Liter
N- Hexane Product Specifications
- For Lab
- Liquid Coating
- N- Hexane
- 99%
- 110-54-3
N- Hexane Trade Information
- Paypal Cash Advance (CA) Telegraphic Transfer (T/T) Cash in Advance (CID)
- 200 Liter Per Day
- 2-5 Days
- Central America Middle East South America Western Europe Asia Eastern Europe North America Australia Africa
- All India
Product Description
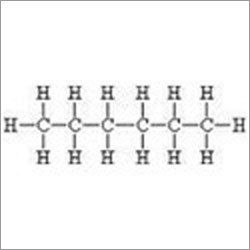
With the constant support of our team of robust experts, we are engaged in offering a huge amount of N-Hexane. This high grade N-Hexane is a colorless liquid with a slightly disagreeable odor. The high grade fluid is formed by the straight-chain alkane with six carbon atoms. It evaporates very easily into the air and dissolves and is highly flammable. This liquid is insoluble in water and miscible with alcohol, chloroform, and ether. Used primarily to produce solvents when it is mixed with similar chemicals, this fluid is very popular in the markets.
# N-Hexane Properties:
1. Chemical Formula: C6H14
2. Molecular Weight: 86.18 g/mol
3. Physical State: N-hexane is a colorless, odorless liquid at room temperature.
4. Boiling Point: The boiling point of n-hexane is approximately 68.7 degree centigrade. This relatively low boiling point makes it volatile and
easily evaporates.
5. Melting Point: N-hexane has a melting point of around -95 degree centigrade.
6. Density: The density of n-hexane at 20 degree centigrade is about 0.660 g/cm3.
7. Solubility: N-hexane is not very soluble in water, as it is a nonpolar compound. It is, however, soluble in many organic solvents.
8. Odor: It is relatively odorless, but the chemical's fumes can be detected at high concentrations, and it is flammable.
9. Flammability: N-hexane is highly flammable and poses a fire hazard. It has a wide flammable range in air (1.15% to 7.8% by volume).
10. Reactivity: It is relatively unreactive under normal conditions, but it can react with strong oxidizing agents.
# Uses of N-Hexane:
Solvent: N-hexane is commonly used as a non-polar solvent in various industries, such as in the extraction of vegetable oils, as a cleaning agent, and in the production of rubber and adhesives.
In some regions, n-hexane has been used as a fuel component, but it is less common for this purpose due to safety and environmental concerns.
# N- Hexane FAQ:
Q. What is n-hexane?
Ans: N-hexane, also known as normal hexane, is a hydrocarbon with the chemical formula C6H14. It is a straight-chain alkane and is one of the isomers of hexane.
Q. What are the common uses of n-hexane?
Ans: N-hexane is primarily used as a non-polar solvent. It is commonly employed in the extraction of vegetable oils, as a cleaning agent, and in the production of rubber, adhesives, and various industrial processes.
Q. Is n-hexane flammable?
Ans: Yes, n-hexane is highly flammable. It has a wide flammable range in air, making it a fire hazard. It's important to store and handle n-hexane with care in well-ventilated areas, away from open flames, sparks, or other ignition sources.
Q. Is n-hexane toxic?
Ans: Prolonged or repeated exposure to n-hexane vapor or liquid can have adverse health effects, particularly on the nervous system. It is important to use proper safety precautions, including personal protective equipment, when working with n-hexane.
Q. What are the health risks associated with n-hexane exposure?
Ans: Health risks can include neurotoxic effects, such as peripheral neuropathy, with symptoms like numbness, tingling, and muscle weakness in the extremities. These effects can be long-lasting if exposure is not managed properly.
Q. Is n-hexane soluble in water?
Ans: N-hexane is not very soluble in water because it is a nonpolar compound. It is, however, soluble in many organic solvents.
Q. What precautions should be taken when working with n-hexane?
Ans: When working with n-hexane, it's essential to do so in well ventilated areas to prevent the buildup of vapors. Proper personal protective equipment, including gloves and safety goggles, should be worn. Open flames, sparks, and heat sources should be avoided in the vicinity.
Q. Can n-hexane be used as a fuel?
Ans: In some regions, n-hexane has been used as a fuel component, but it is less common for this purpose today due to safety and environmental concerns. It is more widely used as a solvent.
Q. What is the difference between n-hexane and other isomers of hexane?
Ans: N-hexane is one of the isomers of hexane. The primary difference between n-hexane and other isomers lies in their molecular structures. N-hexane has a straight-chain structure, while other isomers have branched or cyclical structures. This affects their physical and chemical properties.
Q. Where can I find n-hexane for industrial or laboratory use?
Ans: N-hexane can typically be purchased from chemical suppliers or distributors that cater to industrial and laboratory needs. It is often available in different purity grades depending on the specific application.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Aliphatic Hydrocarbon Solvents' category
MEHTA PETRO-REFINERIES LIMITED
GST : 27AAACM5768N2ZT
GST : 27AAACM5768N2ZT
509, HIVE 67, ICON Tower, Borsapada Road, Next to Raghuleela Mall, Kandivali (West).Mumbai - 400067, Maharashtra, India
Phone :08045812121
 |
MEHTA PETRO REFINERIES LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese






